Typically the ships that I get to sail on are never too far away from shore and a place to tie up. This means quite often it easy for us to restock on fuel, food, water, and other supplies.
But there are also trips, or situations, were we go through a lot of our potable water, aka the water I drink everyday to prevent myself from turning into a desert.
A lot of the potable water gets used in the showers, sinks, miscellaneous cleaning throughout the vessel, and the galley for cooking and washing.
To be on the safe side, it always a good idea to make water on board the vessel whenever possible.
So, how do you make it so that sea water is okay to drink?
On board we have two means available to make potable water when we are at sea. We have a Reverse Osmosis (RO) watermaker and an Evaporator. This post will cover how an RO works, and hopefully if my lack of sleep hasn’t caught up to me, I’ll cover the evaporator in a second post later on.
Seawater contains many tiny particles called known as total dissolved solids (TDS). These dissolved solids include particles of: salt, calcium, magnesium, sulfate, and silica. The quantity of these solids in seawater is anywhere from 35000 – 38000 parts per million (ppm). Just to give you an idea, the typical number of TDS in shore based water in anywhere from 500-1000 ppm.
To reduce the number of TDS in the seawater a high-pressure pump passes pressurized seawater over a semi-permeable membrane. This membrane has tiny tiny holes in it that will allow water molecules to pass through, but blocks the passage of other, larger particles. Occasionally, these larger particles are flushed off the membrane and overboard, using available seawater. The water molecules that do pass through the membrane are pumped into our potable water tanks. A conductivity meter measures the amount of TDS in the produced water and if it is deemed to be too high, this water is diverted overboard.
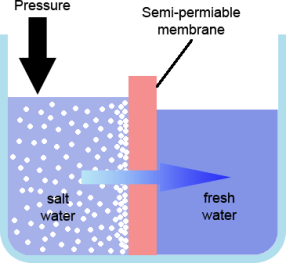
The rate at which water is created is dependent on the pressure of the water being fed to the membranes. A greater pressure = greater water production. However, too high a pressure and the membranes can become damaged. The temperature of the incoming seawater also makes a difference in water production. The ideal temperature is 25°C, though after passing through a heater our seawater typically enters the RO at 16°C.
Originally when I started writing this post I was under the impression that the RO didn’t actually create water that quickly. However, when I checked the numbers it appears that it creates 400L of drinking water in an average of six hours, which is way more than I thought; now I have even more respect for what the RO can do.